Description
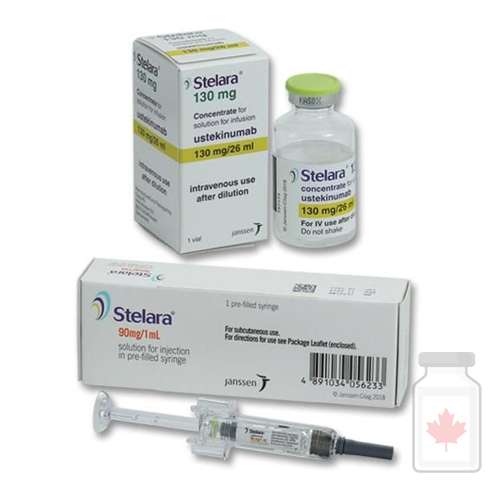
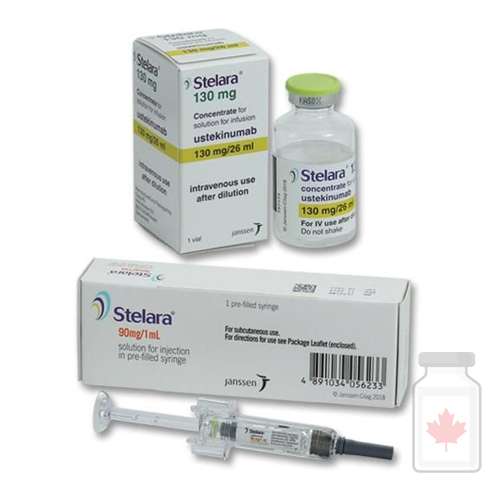
Stelara Ustekinumab
Stelara Ustekinumab is a biologic medication used to treat certain chronic autoimmune and inflammatory conditions. Classified as a monoclonal antibody, Stelara Ustekinumab works by targeting specific proteins in the immune system that drive inflammation.
Stelara is approved for conditions such as plaque psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. It is administered via subcutaneous injection or intravenous infusion (for initial dosing in Crohn’s and ulcerative colitis).
How Stelara Ustekinumab Works
Stelara is unique among biologics because it targets two interleukins:
-
IL-12 and IL-23, proteins that play key roles in the inflammatory process.
By blocking these cytokines, Stelara:
-
Reduces inflammation and immune overactivity.
-
Helps clear psoriasis plaques.
-
Controls joint inflammation in psoriatic arthritis.
-
Induces and maintains remission in Crohn’s disease and ulcerative colitis.
This dual inhibition makes Stelara effective for multiple autoimmune conditions.
Approved Medical Uses of Stelara
Stelara is FDA- and EMA-approved for:
-
Plaque Psoriasis (moderate to severe): For adults and children 6+ years old.
-
Psoriatic Arthritis (PsA): For adults with active joint and skin involvement.
-
Crohn’s Disease (CD): For adults with moderate to severe disease not responding to other treatments.
-
Ulcerative Colitis (UC): For adults with moderate to severe disease.
Dosage and Administration
-
Form: Prefilled syringes, autoinjectors, or vials for injection/infusion.
-
Route:
-
Subcutaneous injection (skin) for psoriasis and psoriatic arthritis.
-
Intravenous infusion (initial dose) followed by subcutaneous injections for Crohn’s disease and ulcerative colitis.
-
-
Common Schedule:
-
Initial dose → Follow-up dose at 4 weeks → Maintenance dose every 8–12 weeks.
-
⚠️ Dosage depends on patient weight, condition, and physician’s guidance.













Reviews
There are no reviews yet.