Description
Enbrel Etanercept, is a biologic medication used to treat chronic autoimmune and inflammatory diseases. Classified as a tumor necrosis factor (TNF) inhibitor, Etanercept works by reducing excessive immune activity that leads to inflammation and tissue damage.
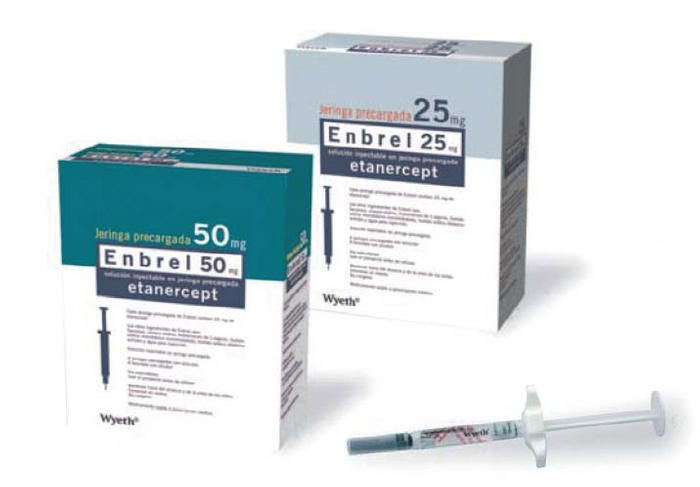
Enbrel is commonly prescribed for conditions such as rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis. It is administered as a subcutaneous injection, often using prefilled syringes or autoinjector pens for at-home use.
How Enbrel Etanercept Works
Enbrel Etanercept targets TNF-alpha, a protein responsible for promoting inflammation in autoimmune disorders. By binding to TNF-alpha, Etanercept:
-
Reduces inflammation in joints, skin, and other tissues.
-
Prevents disease progression by protecting against structural joint damage.
-
Improves quality of life by controlling pain, stiffness, and swelling.
Unlike some biologics, Etanercept is a fusion protein that mimics the body’s natural TNF receptors, acting as a decoy to block inflammatory signals.
Approved Medical Uses of Etanercept (Enbrel)
Enbrel is FDA- and EMA-approved for several autoimmune conditions, including:
-
Rheumatoid Arthritis (RA): Reduces joint pain, swelling, and long-term damage.
-
Psoriatic Arthritis: Controls joint inflammation and skin symptoms.
-
Ankylosing Spondylitis (AS): Improves mobility and reduces spinal inflammation.
-
Plaque Psoriasis: Clears skin lesions and reduces severity of flare-ups.
-
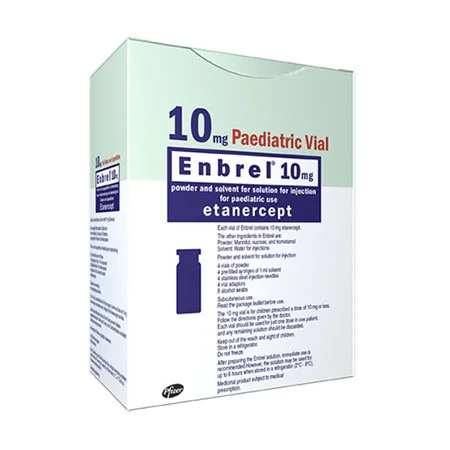
Polyarticular Juvenile Idiopathic Arthritis (JIA): Approved for children ages 2 and older with persistent arthritis.
Dosage and Administration
-
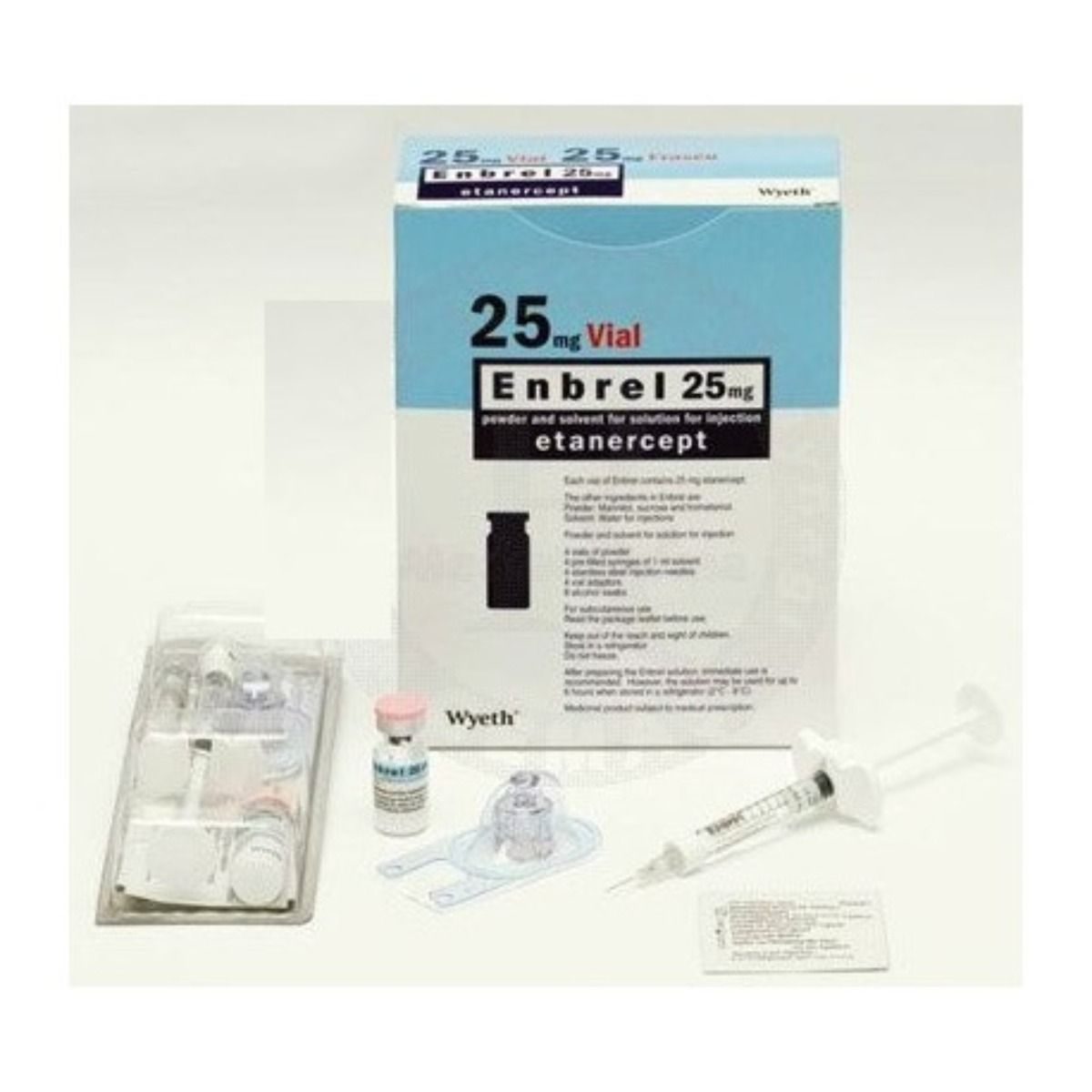
Form: Prefilled syringes, autoinjector pens, and powder vials for subcutaneous injection.
-
Common Dosage:
-
Adults: 50 mg once weekly, or 25 mg twice weekly.
-
Children (JIA): Weight-based dosing.
-
-
Self-Administration: Patients are trained to inject into the thigh, abdomen, or upper arm.
⚠️ Dosage may vary based on age, weight, condition severity, and physician guidance.









Reviews
There are no reviews yet.